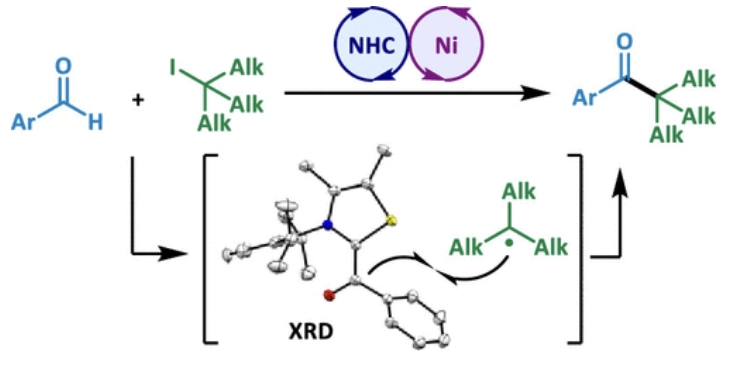
Boosting NHC Radical Organocatalysis with Nickel Chemistry: A Rational Mechanistic Study-based Approach
L. Delfau, E. Mauro, J. Pecaut, D. Martin, E. Tomás-Mendivil
ACS Catal. 2024, 14, 7149−7156, DOI: 10.1021/acscatal.4c01253

Improved protocols for the synthesis of Precursors of Thiazol-2-ylidene N-Heterocyclic Carbenes
L. Delfau, E. Mauro, J. Pecaut, D. Martin, E. Tomás-Mendivil
Synlett 2024, asap ; DOI: 10.1055/a-2284-4798
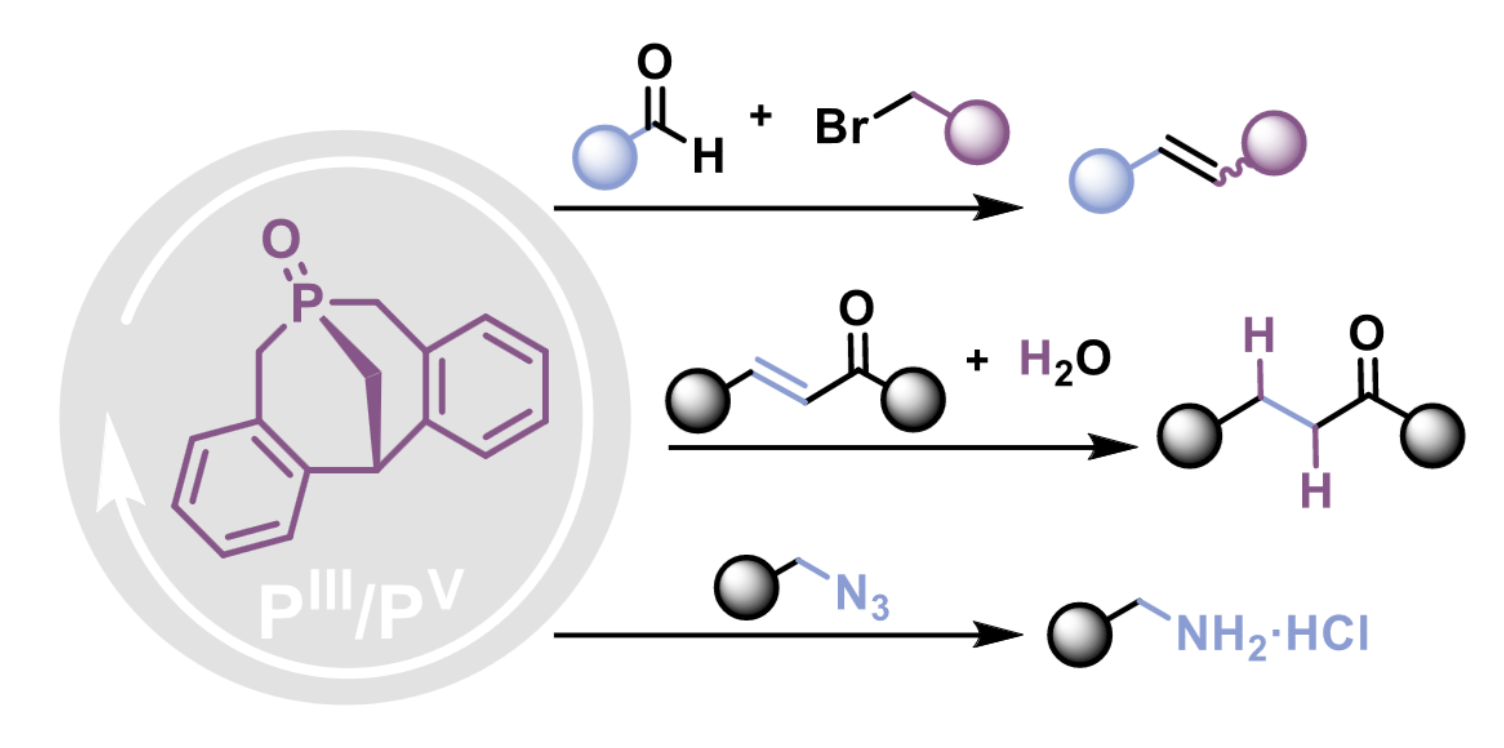
Benchmarking methanophosphocines as versatile PIII/PV redox organocatalysts
L. Mele, R. El Bekri Saudain, J.-L. Pirat, E. Tomás-Mendivil, D. Martin, D. Virieux, T. Ayad
Adv. Synth. Cat. 2024, just accepted ; DOI: 10.1002/adsc.202400468

On the Redox Properties of the Dimers of Thiazol-2-ylidenes That Are Relevant for Radical Catalysis.
L. Delfau, N. Assani, S. Nichilo, J. Pecaut, C. Philouze, J. Broggi, D. Martin, E. Tomás-Mendivil
ACS Org. Inorg. Au 2023, 136–142 ; DOI: 10.1021/acsorginorgau.3c00008

Bending Enamine Patterns of Stabilized Pentalenes into “Polymethine Ylides”.
V. Thery, C. Barra, A. Simeoni, J. Pecaut, E. Tomás-Mendivil, D. Martin
Organic Letters 2023, 25, 560–564; DOI: 10.1021/acs.orglett.3c00037
An air-stable radical with a redox-chameleonic amide.
J. L. Peltier, M. R. Serrato, V. Thery, J. Pecaut, Eder Tomás-Mendivil, G. Bertrand, R. Jazzar, D. Martin
Chemical Communications 2023, 59, 595–598; DOI: 10.1039/d2cc05404c

Beyond the limits of atropochirality: design of highly conformationally restrained biaryls with bridgehead phosphine oxide.
L. Mele, R. Babouri, J.-L. Pirat, E. Tomás-Mendivil, D. Martin, T. Ayad, D. Virieux
Chem. Eur. J. 2023, 29, e202300452; DOI: 10.1002/chem.202300452

The curious case of a sterically crowded Stenhouse salt.
V. Thery, F. Molton, S. Sirach, N. Tillet, J. Pecaut, E. Tomás-Mendivil, D. Martin,
Chemical Science 2022, 13, 9755–9760; DOI: 10.1039/D2SC01895K

Critical Assessment of the Reducing Ability of Breslow-type Derivatives and Implications for Carbene-catalyzed Radical Reactions.
L. Delfau, S. Nichilo, F. Molton, J. Broggi, E. Tomás-Mendivil, D. Martin,
Angewandte Chemie Int. Ed. 2021, 60, 26783–26789; DOI: 10.1002/anie.202111988

Absolute Templating of M(111) Cluster Surrogates by Galvanic Exchange
J. L. Peltier, M. Soleilhavoup, D. Martin, R. Jazzar, G. Bertrand
Journal of the American Chemical Society 2020, 142, 16479-16485; DOI : 10.1021/jacs.0c07990
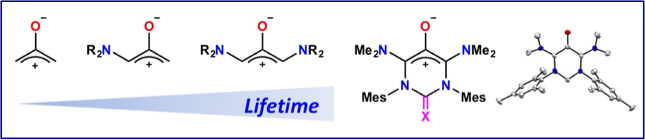
Air-stable Oxyallyl Patterns and a Switchable N-Heterocyclic Carbene.
E. Tomás-Mendivil, M. Devillard, V. Regnier, J. Pecaut, D. Martin
Angewandte Chemie Int. Ed. 2020, 59, 11516–11520; DOI: 10.1002/anie.202002669

Synthesis of dicationic dioxolium salts and their fate upon one-electron reduction.
M. Devillard, V. Regnier, J. Pecaut, D. Martin
Org. Chem. Front., 2019, 6, 3184–3191; DOI: 10.1039/c9qo00298g

What are the Radical Intermediates in Oxidative N-Heterocyclic Carbene Organocatalysis ?
V. Regnier, E. A. Romero, F. Molton, R. Jazzar, G. Bertrand, D. Martin
J. Am. Chem. Soc., 2019, 141, 1109–1117; DOI :10.1021/jacs.8b11824

Metal free oxidation of vinamidine derivatives and a simple synthesis of a-keto-ß-diimines ligands
M. Tripathi, V. Regnier, Z. Ziani, M. Devillard, C. Philouze, D. Martin
RSC Advances, 2018, 8, 38346–38350; DOI: 10.1039/c8ra08220k
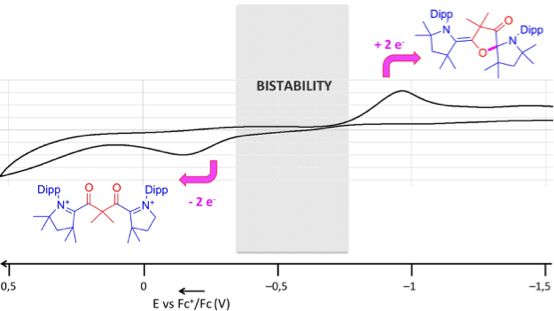
The Serendipitous Discovery of a Readily Available Redox-Bistable Molecule Derived from Cyclic(alkyl)(amino)carbenes
J. K. Mahoney, V. Regnier, E. A. Romero, F. Molton, G. Royal, R. Jazzar, D. Martin, G. Bertrand
Organic Chemistry Frontiers, 2018, 5, 2073-2078; DOI: 10.1039/C8QO00447A

A computational study of the interplay of steric and electronic effects in the stabilization of 1,3-(diamino)oxyallyls.
M. Devillard, V. Regnier, M. Tripathi, D. Martin
J. Mol. Struct. 2018, 1172, 3–7; DOI: 10.1016/j.molstruc.2018.02.029
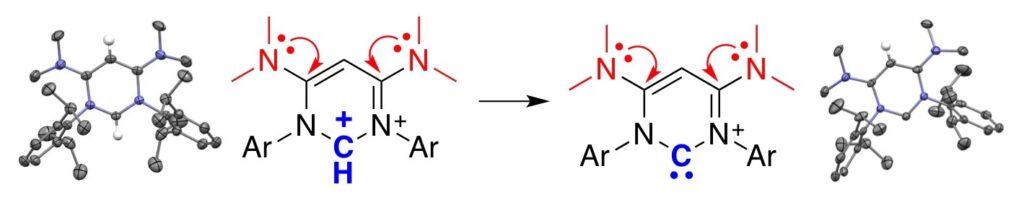
Stable di- and tri-coordinated carbon(II) supported by an electron-rich β-diketiminate ligand.
V. Regnier, Y. Planet, C. E. Moore, J. Pecaut, C. Philouze, D. Martin
Angew. Chem. Int. Ed. 2017, 56, 1031–1035; DOI: 10.1002/anie.201610798
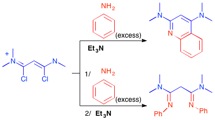
Investigation of the full reversal of selectivity in the reaction of aniline with 1,3-dichloro-1,3-bis(dimethylamino)vinamidinium salts.
M. Tripathi, V. Regnier, C. Lincheneau, D. Martin
New. J. Chem. 2017, 41, 15016–15020; DOI: 10.1039/C7NJ03442C

On the Advantage of Cyclic over Acyclic Carbenes to Access Isolable Capto-Dative C-Centered Radicals.
J. K. Mahoney, R. Jazzar, G. Royal, D. Martin, G. Bertrand
Chem. Eur. J., 2017, 23, 6206–6212; DOI: 10.1002/chem.201700144
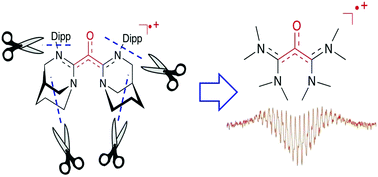
A persistent oxylallyl radical cation with simple di(methyl)amino substituents.
V. Regnier, F. Molton, C. Philouze, D. Martin
Chem. Commun. 2016, 52, 11422–11425.; DOI: 10.1039/C6CC06260A
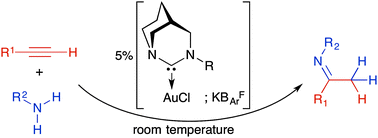
Room temperature hydroamination of alkynes with anilines catalyzed by anti-Bredt di(amino)carbene gold(I) complexes.
X. Hu, D. Martin, G. Bertrand
New J. Chem., 2016, 40, 5993–5996; DOI: 10.1039/C6NJ00980H

A Ruthenium Catalyst for Olefin Metathesis Featuring an Anti-Bredt N-Heterocyclic Carbene Ligand.
D. Martin, V. M. Marx, Robert H. Grubbs, G. Bertrand
Adv. Synth. Catal. 2016, 358, 965–969; DOI: 10.1002/adsc.201501140

The quest for observation and isolation of oxyallyl derivatives.
V. Regnier, D. Martin
Organic Chemistry Frontiers, 2015, 2, 1536–1545; DOI: 10.1039/C5QO00230C
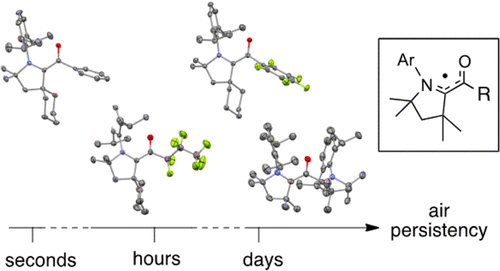
Air-persistent Monomeric (Amino)(carboxy) Radicals Derived from CAACs.
J. K. Mahoney, D. Martin, F. Thomas, C. Moore, A. L. Rheingold, G. Bertrand
J. Am. Chem. Soc. 2015, 137, 7519–7525; DOI: 10.1021/jacs.5b04414
